Mysteries
Mysteries  Mysteries
Mysteries  History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
 Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Space
Space Ten Astonishing New Insights into Alien Worlds
 Weird Stuff
Weird Stuff 10 Bizarre Summer Solstice Rituals Still Practiced Today
 Mysteries
Mysteries Top 10 Haunting Facts About the Ghost Ship MV Alta
 History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
Who's Behind Listverse?

Jamie Frater
Head Editor
Jamie founded Listverse due to an insatiable desire to share fascinating, obscure, and bizarre facts. He has been a guest speaker on numerous national radio and television stations and is a five time published author.
More About Us Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Space
Space Ten Astonishing New Insights into Alien Worlds
 Weird Stuff
Weird Stuff 10 Bizarre Summer Solstice Rituals Still Practiced Today
10 Nuclear Mysteries We’ve Managed To Solve
For better or worse, nuclear energy has changed the world. But it’s not without its mysteries. Since nuclear energy was discovered, it has given rise to a number of perplexing questions. Many have been answered, but many others only create more mysteries.
10 The Rope Trick Effect
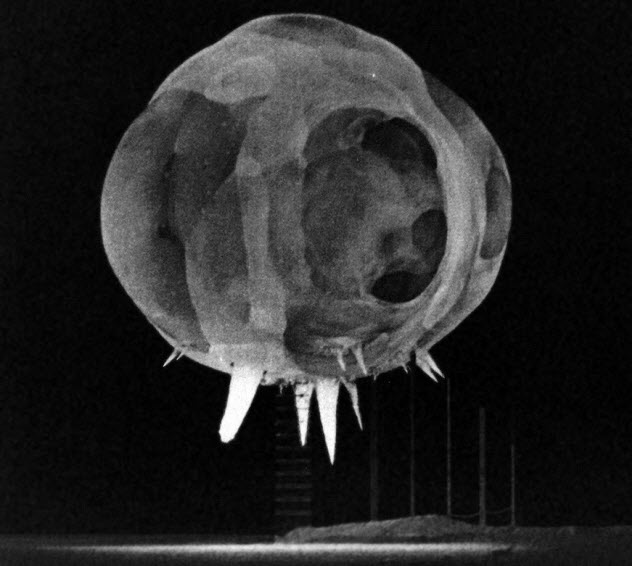
In the 1940s and ’50s, scientists were trying to understand nuclear explosions by taking pictures of them just milliseconds after a bomb went off. Immediately, they noticed bizarre spikes protruding from the bottom. They assumed nuclear blasts would be mostly symmetrical, so the strange spikes were a complete mystery.
A researcher named John Malik investigated the odd phenomenon. He soon noticed that the spikes were in the same place as the cables holding the bomb in place on a tower. Malik presumed the cables created the strange spikes, but he had to test his theory. During the next few explosions, he painted the cords with different types of paint. He even tried aluminum foil. In the ensuing photos, the spikes indeed proved to be the cables. When photographed, though, they had a reverse color like a negative photo.
Black cables appeared white and light ones, dark. As dark shades absorb more heat than light shades, the dark-colored cables absorbed the blast’s heat and vaporized in a bright white light. Light-colored ones did not absorb heat as fast and didn’t glow. With the mystery solved, Malik named the phenomenon the “Rope Trick Effect.”
9 Radioactive Rain
After the meltdown at the Fukushima Daiichi nuclear reactor and the news that radioactive debris was in the Pacific, some residents of the North American West Coast were concerned about radiation drifting their way. Several YouTube videos showed Geiger counters recording unnaturally high levels of radiation after rain. The connection to Fukushima was made, as were conspiracy theories about government cover-ups.
Despite the confusion that may be generated by videos like these, experts say that an occasional radiation spike after rain is a naturally occurring phenomenon. A large amount of uranium is present in soil and rock and goes through a number of chemical changes throughout its half-life of 4.5 billion years. Eventually, it turns into radon gas, which then seeps out of the soil. Sometimes, there are phenomena called radon washouts, where the naturally accumulated radon falls to Earth in precipitation. Radon has a half-life of only a few days, so the radiation soon clears up and is not considered a health risk.
8 Why Is There So Much Lithium?

The lithium question has bothered scientists for years. There’s a lot of lithium in the universe, but no one’s been able to explain why. Most heavy elements in the universe form inside of stars and through supernovae, but lithium-7 can’t withstand those kinds of temperatures.
Lithium is a “light element” that can’t form inside of stars. It’s much less abundant in the Milky Way than nearby elements in the periodic table. While some lithium was left over from the Big Bang and cosmic rays interacting with interstellar matter may have formed more, it still doesn’t explain the amounts we have measured in the universe.
In the 1950s, scientists theorized that beryllium-7 sometimes formed near the surface of a star and was then pushed to its outer regions where it decayed into lithium. But no one knew for sure until Japan’s Subaru Telescope witnessed Nova Delphini 2013. After 60 years, astronomers were finally able to put the mystery to rest when they detected beryllium being shot out of the exploding star at high velocity—the perfect scenario for creating lithium.
The solutions to these kinds of space mysteries, however, often lead to more questions. After the beryllium was observed, it just disappeared, leaving scientists scratching their heads as to where it all went so suddenly.
7 The Project Faultless Mystery

In the Nevada desert, there is a cylinder 2.5 meters (8 ft) tall that marks the site of Project Faultless, the underground detonation of a nuclear bomb on January 19, 1968. As sites were used repeatedly for testing, it was extremely unusual that this one had only a single detonation.
So why did the government build an expensive underground nuclear testing facility just for a single bomb? During the Cold War, both sides detonated countless devices in the arms race. At one time, Las Vegas was shaking with a new explosion every three days. Business owners were getting tired of the testing, but one in particular, multibillionaire Howard Hughes, had more pull than the others.
After enduring the shaking for long enough, Hughes wrote a long, rambling letter to President Lyndon Johnson complaining about the explosions. It was thought that his letter was ignored, but it turns out not even the president can ignore one of the richest and most powerful men in the world. In addition to controlling Las Vegas, Hughes was an oil magnate and one the largest defense contractors in the US. Eventually, Johnson caved to pressure from Hughes and initiated Project Faultless to see if moving the testing site farther away from Vegas would solve the shaking problem.
Faultless was one of the largest hydrogen bombs ever detonated on the US mainland. The blast was so strong that it caused the ground to sink 2.5 meters (8 ft) and open fissures 1 meter (3 ft) across. But despite having the testing facility moved, it didn’t do anything to alleviate the shaking in Vegas, much to the disappointment of Hughes and the city’s hotel owners.
6 Japan’s Radioactive Mushrooms

During the Fukushima disaster, radiation spread over a good portion of northeast Japan. While food from Fukushima was mostly restricted due to its high radiation content, most food from the surrounding prefectures was found to have either normal levels of radiation or levels within strict limits. However, picking and eating wild mushrooms is a pastime in Japan. After the disaster, many of the wild mushrooms even hundreds of kilometers away were found to have radiation levels far over legal limits.
Some mushrooms are radiation magnets. They are so good at sucking up radiation that they’ve even been proposed as a way to clean up radiation from fallout. When mushrooms with high levels of radiation were discovered in Japan, the government slapped a blanket ban on selling all kinds of wild mushrooms in stores and restaurants unless they were tested and found to be safe.
However, a mystery soon arose. After testing, some of the mushrooms with levels that exceeded the legal limit were found to have radiation that couldn’t have come from the failed plant. So the question was: Where was it from?
Tests revealed that the radiation was actually much older. The type of radiation these mushrooms contained was from nuclear tests from the 1940s, ’50s, and ’60s. Some was also traced to the Chernobyl disaster. Although the area where the mushrooms were picked was safe, the mushrooms had absorbed the lingering radiation, which then accumulated to dangerous levels. The radiation absorption rate of mushrooms differs from species to species. But with most people unable to tell which kinds of mushrooms pose a contamination risk, researchers recommended not eating self-picked mushrooms after the discovery.
5 Manganese’s Unexplained Decay Rate
In 2006, physicists at Purdue, Stanford, and other locations recorded a phenomenon that spit in the face of modern nuclear science. Radioactive decay rates have long been held to be constant, but these researchers found that radioactive decay rates grew more pronounced in winter than in summer. Naturally, they tested the unusual findings in several different labs to check for errors but found the results were constant. Their search for an explanation took them away from our planet and toward the Sun.
When checking the decay rate of a manganese isotope, a Purdue physicist found that the change in rates coincided with a solar flare that happened one night earlier. From 2006 to 2012, the unusual occurrence was recorded during 10 solar flares.
While physicists have solved why the decay rate of manganese-54 mysteriously changed, they have not figured out the science behind it. They believe it may be an interaction between ionizing particles and neutrinos, but it’s difficult to be sure. Regardless of why it happens, this discovery may be used to create a warning device for solar eruptions. Purdue has already filed a patent for the concept, which could provide a timely warning to shut down power plants and communication infrastructures before a coronal mass injection has devastating consequences on modern technology.
4 China’s Nuclear Raid On South Africa
In 2007, two groups of armed men raided the Pelindaba Nuclear Research Center in South Africa. They deactivated layers of security and injured an off-duty night guard, finally succeeding in stealing a laptop from the facility’s control room. They were never apprehended.
After the break-in, conspiracy theories abounded as to the identity of the culprits. Officially, the South African government labeled the break-in as a failed burglary. But it didn’t answer why two groups of burglars would raid a nuclear facility only to steal a laptop. Picking up on the shakiness of the “burglary” theory, several US media outlets pounced upon the incident and labeled it as a terrorist group’s attempt to build a nuclear weapon.
Wikileaks released a series of diplomatic cables between the US and South Africa in which the South African government stuck to its burglary theory. Later, though, documents leaked to Al Jazeera claimed that South African spies laid the blame on the Chinese government, which later instituted a nuclear program using the same type of technology used at Pelindaba.
3 The Radiation Cloud Over Europe

In 2011, the Czech Republic’s Office of Nuclear Safety recorded an uptick in radiation across the country. Soon after, organizations throughout Europe began getting hits from iodine-131, a by-product of nuclear reactors and nuclear weapons. Given that it was shortly after Fukushima, the public’s sights went immediately to Japan as the culprit. However, much like the radon washouts, the connection was again put to rest by scientists. As the Fukushima meltdown would have released several other types of isotopes besides those detected by scientists, the source of the radiation was a mystery.
Theories abounded. Some said it started in a pharmaceutical manufacturing plant. Others said it could have leaked from a hospital. Still others said it could have come from a nuclear submarine or a leak while transporting nuclear materials. Eventually, Hungary said the source was probably released from the Institute of Isotopes Co., Ltd., an isotope maker in Budapest that produces materials for healthcare, research, and industry. The mystery seemed to have been cleared up, though the director of the institute said that the amount detected was beyond what his institute could have emitted.
Regardless of where it came from, the levels of radiation detected were just a 40,000th (or .0025 percent) of the dose of a transatlantic flight. But while it was not high enough to pose a risk to human health, news of a radioactive cloud spreading across Europe was no doubt unsettling for its residents.
2 The 1,200-Year-Old Nuclear Mystery Solved By An Undergrad And Google

By studying tree ring data, scientists discovered that Earth was hit by an intense burst of high-energy radiation 1,200 years ago. Around 774 to 775, the level of the radioactive isotope carbon-14 increased by 1.2 percent, which doesn’t sound like much but is about 20 times the normal level of radiation. This kind of change could only have been caused by a supernova or solar storm from a giant solar flare. However, the effects of such an event would have been noticed at the time, and historical records appeared to show nothing.
Then Jonathon Allen, a biochemistry major at the University of California, listened to a Nature podcast detailing the find. Unlike the other researchers, he tried a simple Google search. It brought him to the Avalon Project, a library of online legal and historical documents. Scrolling through a copy of the eighth-century Anglo-Saxon Chronicle, he found reference to a “red crucifix” that appeared in the heavens “after sunset.”
It could easily have been an unrecorded supernova. The object was seen in the western skies after sunset and may have been obscured by the Sun, explaining why it went unrecorded. It may have also been further obscured by a dense cloud of interstellar dust, which would explain the red hue. As it’s dealing with events that happened over 1,000 years ago, the mystery will never be solved to the satisfaction of everyone, but Allen’s idea has impressed many scientists.
1 Why Is Red Paint So Cheap?

Why red paint is cheaper than other colors is not something normally associated with nuclear fusion. Nonetheless, it is a nuclear mystery. Red ocher, Fe2O3, is an iron compound that makes paint red. It’s cheap compared to other color compounds because it’s so plentiful, and interstellar nuclear fusion is the reason there’s so much of it.
A star goes through various stages of nuclear fission, shrinking as its power level dissipates. But as it gets smaller, its pressure increases, which also causes a rise in temperature. This creates more reactions, which in turn form more heavy elements. It’s a cycle that repeats throughout a star’s lifetime, creating more heavy elements farther up the periodic table.
The process continues until the total number of protons and neutrons reaches 56, at which time the star collapses. As 56 is the end of the cycle, stars produce more things with 56 neucleons (aside from super light elements) than anything else. Iron, which is used to make red paint, has 56 neucleons in its stable state. So, red paint is cheap because it is a product of the universe’s billions of dead stars.
Nathan keeps a Japan blog where he writes about the sights and expat life and finds Japanese culture in everyday items. You can also find him on Facebook and Twitter.