 Mysteries
Mysteries  Mysteries
Mysteries  History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
 Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Space
Space Ten Astonishing New Insights into Alien Worlds
 Weird Stuff
Weird Stuff 10 Bizarre Summer Solstice Rituals Still Practiced Today
 Mysteries
Mysteries Top 10 Haunting Facts About the Ghost Ship MV Alta
 History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
Who's Behind Listverse?

Jamie Frater
Head Editor
Jamie founded Listverse due to an insatiable desire to share fascinating, obscure, and bizarre facts. He has been a guest speaker on numerous national radio and television stations and is a five time published author.
More About Us Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Space
Space Ten Astonishing New Insights into Alien Worlds
 Weird Stuff
Weird Stuff 10 Bizarre Summer Solstice Rituals Still Practiced Today
10 Times Tainted Medical Products Caused Serious Harm
The world isn’t perfect, so there will always be a certain number of tragedies that could be prevented. But in some cases, they are caused by glaring errors that could easily be stopped by the slightest degree of attention. When it comes to medicine and treatment, there are many safeguards to keep tainting and infections from happening. But in the following instances, contamination still happened.
10 Antibiotic-Resistant Duodenoscopes
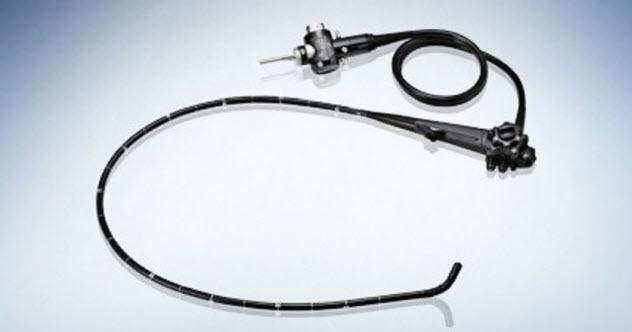
Duodenoscopes are devices that are supposed to drain fluid from pancreatic and bile ducts. However, they are unruly when it comes to sterilization. The scopes have a sort of elevator-like movement which allows them to move within the body, draining fluid as needed. As a result, cleaning the scope is quite laborious. Although any good medical professional would clean such instruments, many did not—which was a mistake that proved deadly.
In 2016, two people in the Chicago area died from a bacterial disease that had been popping up around the United States. From 2012 to 2015, around 250 people were infected with this same illness, which had resulted from a flaw in the same machine: the duodenoscope.
The manufacturers of the scope never actually tested cleaning the device in a real-world setting. In turn, this caused a bacteria to spread that otherwise wouldn’t have developed if the instruments were properly sterilized. The illness that spread was a superbug called carbapenem-resistant Enterobacteriaceae (CRE).
CRE is an incredibly deadly family of bacteria that kills half of the people who become infected and is resistant to even the most potent antibiotics. CRE and similarly drug-resistant E. coli outbreaks occurred throughout the US and even across Europe in France and Germany. Because they failed to disclose the scope’s failings, the three producers—Olympus, Pentax, and Fujifilm—were investigated by the FDA and hospitals were warned not to use these products.
9 Drug-Laced Diet Supplements

In 2013, a dietary supplement known as Dr. Larry’s Tranquility pills were tested by the FDA. The pills were supposed to cause sleep naturally by using substances such as figwort root and licorice. Out of thousands of other pills that claimed similar results, Dr. Larry’s Tranquility pills were tested and found to contain a lot more than claimed. There were powerful prescription drugs in them.
Two powerful sedatives were found in the pills: Thorazine, a potent antipsychotic, and doxepin, an antidepressant and sleeping medication. Dr. Larry was not a doctor at all but a convicted felon. Larry LeGunn, his real name, was a chiropractor whose license was taken away in 2010 for grand theft and insurance fraud.
He was not the only person to use strong drugs to sell diet supplements, though. Jeffrey Bolanos, who had an extensive history with drugs like crack cocaine and methamphetamine, became head of an Arizona company called Beamonstar Products. Three of the company’s sexual enhancement pills actually contained tadalafil, a main ingredient in the prescription drug Cialis. Later, the products were recalled by the company.
In 2009, another company, Kilo Sports, sold “natural” performance-enhancing drugs, only they weren’t natural. They contained steroids, and another was found to have anti-estrogen substances in 2010. Apparently, in 2004, Martin McDermott, the head of Kilo, had several criminal charges filed against him related to felony possession of testosterone, boldenone, and human growth hormones. He apparently used these drugs to illegally “boost” products that he sold over the years.
8 Bayer’s HIV Blood Plasma
In 2003, after an investigation by The New York Times, it was shown that Cutter Biological, a unit of pharmaceutical company Bayer, engaged in controversial business practices in the 1980s that resulted in the spread of HIV among hemophiliacs overseas. Allegedly, in response to complaints about a medicine sold by Cutter, they produced a newer, safer one in 1984. The only problem is that they didn’t stop selling the dangerous, older product in other countries.
The product sold was called Factor VIII Concentrate, and it was produced from 10,000 donors’ blood plasma to help with the treatment of hemophiliacs. However, the donor plasma was not tested for HIV at the time since there was very little awareness of the disease. Because of this, the hemophiliacs who used the product contracted HIV.
In February 1984, the product was reformulated and the old product was supposedly taken off the market. However, according to company records, they continued to sell the drug in Malaysia, Singapore, Indonesia, Japan, and Argentina. Cutter claimed that they did so because customers there doubted the new drug’s effectiveness and a shortage of plasma made creating the new product much harder.
When Hong Kong distributors became interested in the product in late 1984, Cutter told them to use the infected product before using the new formulation. Because of this, around 100 hemophiliacs contracted HIV. The fact that the infected product was still marketed in Asia and other less developed countries caused many customers to accuse Bayer of racial discrimination.
Later, Bayer quietly began to pay off foreign lawsuits related to the product. After the reports became public, Bayer sold their blood plasma business in October 2003.
7 Metal-Tainted Children’s Medication

In May 2009, Johnson & Johnson, the company that manufactures a variety of medications including Children’s Tylenol and Children’s Motrin, began to receive complaints about black specks inside bottles of medication. All the medication had been produced from McNeil’s plant in Fort Washington, Pennsylvania. When the black specks were examined, they were found to be the metals nickel, iron, and chromium.
The medications were liquid and potentially deadly. In April 2010, Johnson & Johnson began a voluntary recall of the medications. The McNeil plant had been plagued with recalls since 2009, and the FDA later sourced the contamination to machinery used during production.
Despite the company discovering the metal particles around this, they continued to produce and sell liquid medication for several months afterward. In 2009, a death occurred due to the tainted medicine: Joshua Arndt, four years old, died after being given one dose of Children’s Tylenol. Although he was rushed to the emergency room, there was nothing that doctors could do to help him.
In 2012, his father filed a lawsuit. But it was dismissed in 2014 because the two-year statute of limitations had passed. Johnson & Johnson did not go without punishment, though. In 2015, charges were brought against the company because they had knowingly sold the medication months after they learned that it was dangerous.
In the end, they had to pay $25 million to settle their legal problems. As for the McNeil plant where the medication was produced, the entire plant was demolished and rebuilt.
6 Deadly Heparin
In 2008, the blood thinner heparin was in such demand in the United States—around 300,000 doses a day—that producers in China began to engage in increasingly shady practices to meet it. Usually, heparin is produced from pig intestines. But according to the FDA, some Chinese producers were making it from cow and sheep intestines, which allowed for the spread of sicknesses unseen in the past.
According to doctors, the main symptom of the contaminated heparin was dangerously low blood pressure, and it was noticed in hospitals and by patients across the United States. By late 2008, 81 deaths had already been linked to the drug. What made this already disturbing situation even worse was the fact that the bad drug made its way through multiple screenings and finally onto the market.
When Baxter International, the distributor of half of all the heparin in the United States, discovered this, they issued an immediate recall. While officials at first believed that only the United States was affected by these contaminations, it became clear that even more countries had received the bad heparin. Eleven countries soon reported similar cases of heparin causing harm.
The contamination was caused by the chemical oversulfated chondroitin sulfate, which is created from non-pig material. Despite Chinese officials claiming that the heparin was not tainted, the FDA managed to trace the heparin to 12 Chinese producers. After this revelation, reforms were made among these companies.
Even though the FDA had been suspicious about Chinese practices since 2007, it wasn’t until 2012 that they began to create serious guidelines. Around 246 deaths have been traced to the heparin since 2007, and it’s believed that some of the contaminated drug may still be on the market nearly a decade later.
5 Bacteria-Infected IV Bags
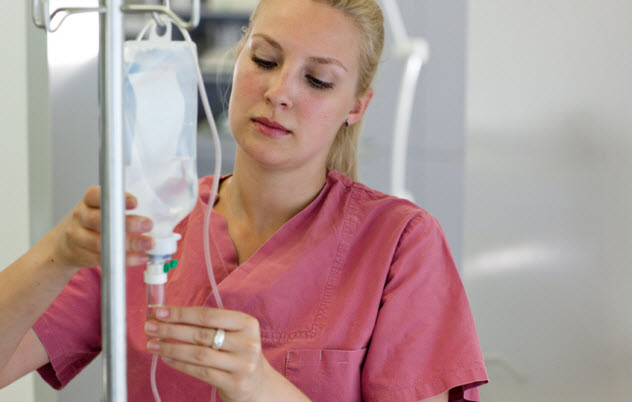
In 2011, 19 people at six hospitals in Alabama became ill for a very specific reason: Their IV bags had been infected by Serratia marcescens bacteremia, a type of bacteria which can prove fatal when it enters the bloodstream. Many of the patients infected by the bacteria were high-risk patients who were given intravenous feeding because they were too sick to eat on their own.
The outbreak began at several Birmingham area hospitals in Alabama in March 2011. Soon, 19 people became ill, and nine of them would eventually die. The bacteria caused almost immediate effects upon entering the bloodstream, including blood pressure and temperature changes. However, once the surviving patients were treated, they managed to recover.
But this wasn’t the only time that infections in IV bags would cause illness in patients. In Minnesota, a series of painkiller thefts by nurses at hospitals would eventually cause infections in the patients. At St. Cloud Hospital, several patients who were supposed to get painkillers were actually given saltwater.
This caused rare bacterial infections in 25 patients. Six of them ended up in intensive care, and one died. Blake Zenner, a nurse who stole painkillers from 2010 to 2011, was found to be responsible for the outbreak and was finally caught in 2012.
4 Meningitis And Mold Steroid Shots
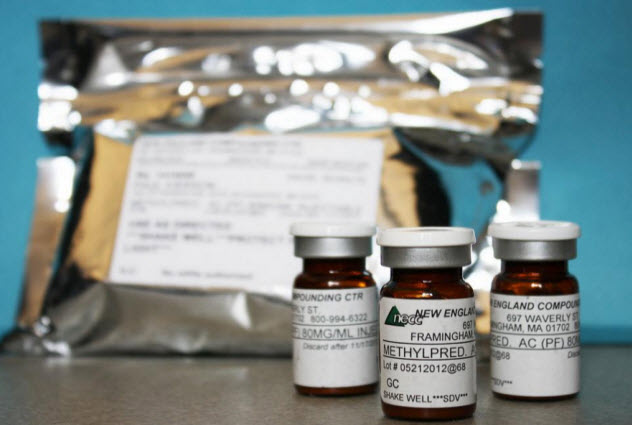
In 2011, the New England Compounding Center began sending out shipments of steroids that were infected with meningitis and fungus. Within a year, the CDC would estimate that 14,000 people had been exposed to the infected steroids. Meningitis could be contracted from the shots along with a mold that could incubate for months. The epidemic spread across 16 states and would infect hundreds of people.
The steroid involved was a formulation of methylprednisolone for injection, and it was tainted by a rare black fungus called Exserohilum rostratum that usually only affects plants. It’s so rare in humans that its incubation wasn’t even known at the time of the outbreak. However, within a year, 268 cases of fungal meningitis, three cases of fungal joint infections, and 21 deaths were reported that were related to the steroid shots.
While most of those with fungal infections reported incubation periods of a few weeks to two months, it was also known that the infections could incubate for several months. So even after the steroid shots were recalled, those who took them could still get sick.
A similar outbreak of fungal meningitis occurred in 2002 with the same type of steroids. This taught valuable lessons about the manufacturing of these substances. Without stringent standards when it comes to mixing them, it was found that fungus grows aggressively, which caused the two outbreaks.
In 2013, the number of fungal meningitis cases was 751 and there were 64 deaths. Even a full year after the outbreak, people still required treatment, which shows just how dangerous a lack of attention can be in the medical world.
3 Deadly Dirty Syringes
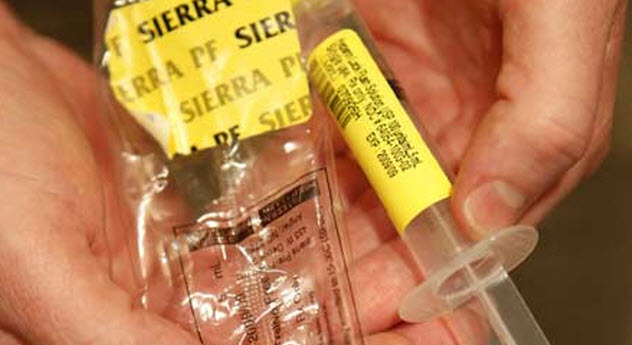
Safety with syringes is commonplace for most patients and professionals: Syringes shouldn’t be reused or shared. But what happens when the people who make the syringes have accidentally contaminated them? This occurred in 2007 when over 100 people became sick with bacterial infections related to the use of contaminated saline syringes that were produced by the same company.
To keep such outbreaks from occurring, there are multiple safety checks that should be put into place by companies. But even after that, the FDA itself examines medical products. In the case of the syringes, evidence shows that the FDA failed to catch the contamination.
The syringes, prefilled with saline, were examined by an FDA inspector before they were shipped out in October 2007. According to the report, the inspector found black, brown, and red particles in the syringes but wrote it off as “rust” and said that the factory management had put a plan in place to take care of it.
Apparently, the factory had switched to an unreliable sterilization method around the time of the FDA inspection, but this wasn’t noted, either. Just one week later, a distributor recalled 1.3 million syringes, which should have caused the FDA to launch another thorough inspection. But they didn’t due to understaffing.
When they finally did another inspection of the factory, it was far below standards and was shut down in January 2008. But by then, the damage was done. Over 100 people had become sick from using the bad syringes, and six actually died. In 2016, B. Braun, the company that sold the syringes, agreed to pay $7.8 million in damages.
2 Bacteria-Infected Ultrasound Gel
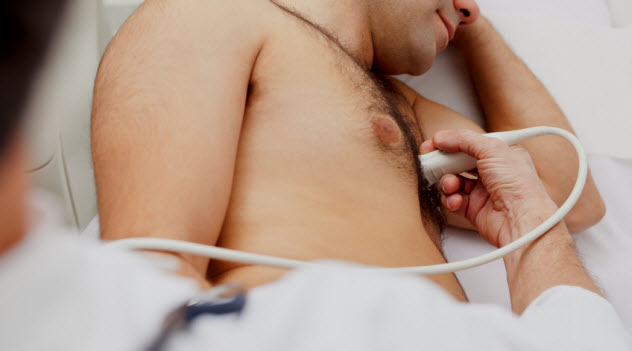
In 2011, doctors in the emergency room at Beaumont Health Center near Detroit began to notice that large numbers of patients were testing positive for the bacteria P. aeruginosa. This particular sort of bacteria is not normal and usually comes from contamination. They began to investigate and found that all 16 patients had mostly identical histories: They became sick with respiratory illnesses after cardiovascular surgeries.
Ultrasound gel is used to improve images for surgeries or exams. In this case, the patients became sick after imaging gel was used on them for surgery and infected their respiratory tracts. In 2008, a study in Europe showed that many imaging bottles tested positive for contamination when cultures were grown.
When the researchers announced their findings at the 18th European Congress of Clinical Microbiology and Infectious Diseases, they warned that bacteria could contaminate gels during the production process. US manufacturers apparently didn’t heed these warnings.
When cultures were grown from the gel bottles used before the outbreak in the United States, they also showed strains of bacteria that came from the manufacturing process. Pharmaceutical Innovations, the New Jersey–based company behind the imaging gel, was raided by US Marshals, and their gel products were seized.
Because imaging gel is so common, it was unknown just how far the contaminated products had been distributed. All the FDA could do was issue a warning that would be known by everyone using the gel because once the gel was applied, the bacteria could infect rapidly. Luckily, no more outbreaks were reported, and new safety standards have since been put in place.
1 Toxic Cough Syrup
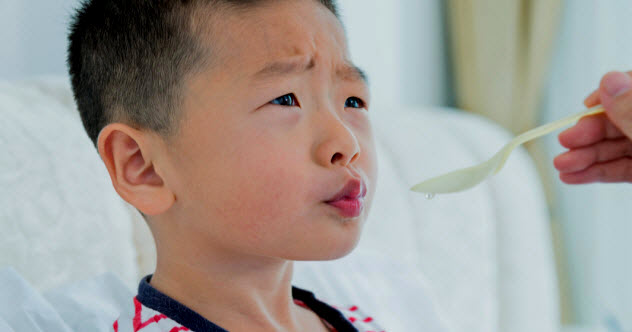
This is one of the most tragic stories of tainted medicine because of how widespread it was and the nature of the people it affected: sick patients in the Third World. It all started with chemical production in China where glycerine, one of the main ingredients in cough syrup, would be substituted with the sweet-tasting but highly deadly chemical diethylene glycol to make extra money.
Diethylene glycol is an industrial solvent and one of the main ingredients in antifreeze. When ingested, it causes kidney failure, paralysis, and finally, multiple organ failure.
Several decades ago, medicine produced with diethylene glycol caused over 100 deaths in the United States, which prompted the FDA to pass strict regulations. But in less developed countries, it has, over two decades, been substituted in a variety of syrups and medicines.
This has caused at least eight mass poisonings with one in Panama resulting in 365 reported deaths. A conservative estimate has put deaths in the thousands. Diethylene glycol first appeared in Bangladesh in 1992 when children died after using counterfeit syrups. Later, 88 children died in Haiti.
All the toxic products can be traced back to the Yangtze delta, colloquially known as “chemical country” by the Chinese because it so well-known for chemical production. There, many raw chemicals are produced and diethylene glycol is often sold in place of glycerol.
In many instances where mass deaths were reported from using these products, they went through multiple inspections by distributors. But none of them discovered the truth. Often, those creating the chemicals weren’t even licensed. But in an effort to make a few extra dollars, buyers will turn a blind eye.
Once they have purchased the product, they will falsely certify it so that distributors will accept it. It took many deaths before the Chinese government finally began to take action. In 2007, the World Health Organization reported that around 440 counterfeit operations had been shut down. Hopefully, these efforts by authorities will be enough to prevent any future tragedies.
Gordon Gora is a struggling author who is desperately trying to make it. He is working on several projects, but until he finishes one, he will write for Listverse for his bread and butter. You can write him at [email protected].








