Travel
Travel  Travel
Travel  Miscellaneous
Miscellaneous 10 Intriguing Origins of Popular Sayings
 Facts
Facts 10 Little Known Facts about the Rubik’s Cube
 The Arts
The Arts Top 10 Iconic Musicals That Got Horrible Reviews
 Misconceptions
Misconceptions Ten Unexpected Truths About How Pirates Really Lived
 Health
Health 10 Most Outrageous Workout Trends
 Weird Stuff
Weird Stuff 10 Surprising Things That Were Designed to Stop Evil Behavior
 Movies and TV
Movies and TV Top 10 Ghost Adventures Episodes That Will Haunt You Forever
 Animals
Animals Ten Animals That Produce and Store Toxins in Unlikely Places
 Weird Stuff
Weird Stuff 10 Weird Things That Warp Your Sense of Time
 Travel
Travel Ten Tiny Kansas Towns with Strange Claims to Fame
 Miscellaneous
Miscellaneous 10 Intriguing Origins of Popular Sayings
 Facts
Facts 10 Little Known Facts about the Rubik’s Cube
Who's Behind Listverse?

Jamie Frater
Head Editor
Jamie founded Listverse due to an insatiable desire to share fascinating, obscure, and bizarre facts. He has been a guest speaker on numerous national radio and television stations and is a five time published author.
More About Us The Arts
The Arts Top 10 Iconic Musicals That Got Horrible Reviews
 Misconceptions
Misconceptions Ten Unexpected Truths About How Pirates Really Lived
 Health
Health 10 Most Outrageous Workout Trends
 Weird Stuff
Weird Stuff 10 Surprising Things That Were Designed to Stop Evil Behavior
 Movies and TV
Movies and TV Top 10 Ghost Adventures Episodes That Will Haunt You Forever
 Animals
Animals Ten Animals That Produce and Store Toxins in Unlikely Places
 Weird Stuff
Weird Stuff 10 Weird Things That Warp Your Sense of Time
10 Oddities Of Reproductive Science
Cloning. IVF. Stem cells. Centuries of studying the egg, the cell, and the embryo have led to amazing advances that benefit humankind. Through the science of reproduction, people have accomplished noble goals, such as overcoming infertility, as well as mundane conveniences, such as better beef.
But science’s progress has shown some bizarre turns and freakish results. It has also raised troubling ethical questions. Below are 10 of these oddities of reproductive science.
10 Baby Hair Lassos And Salamanders

Scientist Hans Spemann found that his standard tools did not work well on his test subjects, very early stage salamander embryos. They were just too slippery. While holding his nine-month-old daughter, Spemann had an idea. He snipped off a lock of his daughter’s hair and returned to the lab.
With tiny nooses of baby hair as tools, he carried out his experiments. In an early experiment, he split the embryos. Against typical theories of the time, the result was independent, fully formed salamanders—artificial twins.
In a later experiment, he constricted a cell into a dumbbell shape and pushed the nucleus into one section of the cell. When the baby-hair noose was loosened, the nucleus slipped back into the formerly nucleus-free part of the cell. Then, that part of the cell divided, essentially making a clone of the developed side of the embryo.
Results varied depending on where he cut through the egg and how he constricted it. If the noose cut through one part of the egg, identical twins formed. But if he cut through another part, only one-half of the embryo developed properly. The other half became just a clump of blood and guts.
If he constricted the egg only a little, a two-headed salamander formed. The two heads fought over food for the same body, and he called them “two egotisms in the place of one.” Spemann kept these monstrous salamanders in the name of research.[1]
9 Urine Clones
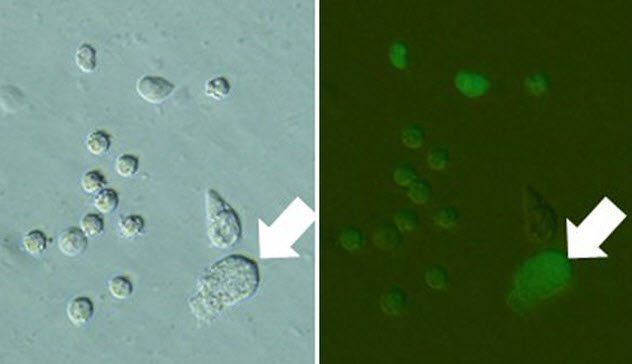
Dolly the sheep was made through nuclear transfer. In this technique, the nucleus from an adult sheep’s cell was put into an egg cell and eventually transferred into a sheep to develop.
Nuclear transfer could be useful in the preservation of endangered mammals. However, taking donor cells has some risk because it might accidentally injure the animal. Researchers from the University of Yamanashi suggest an easy way to collect a variety of donor cells without hurting the animal: Just use urine. Several kinds of cells can be found in urine, such as cells from the bladder and kidney, and these cells can be grown in the lab after collection.
Generally, urine is not good for cell survival. For one thing, some of its ingredients are toxic. For this reason, it was thought that even if live cells could be taken from urine, the environment would affect the cell survival and nucleus integrity.
But this wasn’t a problem. Some of the urine clones survived to an early embryonic stage and were transferred to surrogates to develop further. When those urine clones matured, they still had offspring when bred with each other. The researchers’ tests on the clones’ fertility thus strongly suggest that cells from urine are still good for cloning.[2]
However, a problem still exists. There is only a limited ability to collect the cells in urine from wild animals, especially in clean conditions.
8 IVF In History
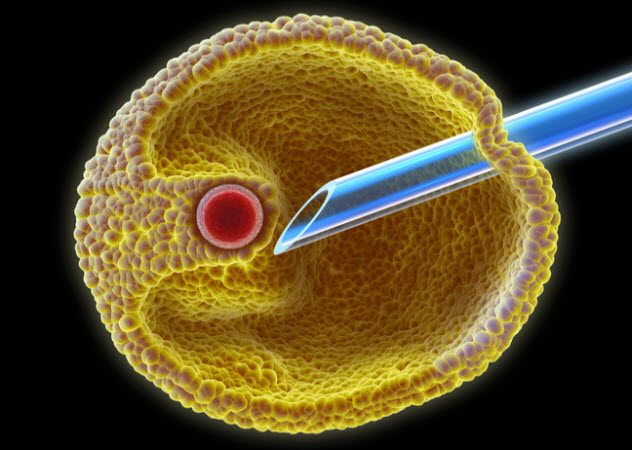
With in vitro fertilization (IVF), infertile couples have a chance to get around their bodies’ limits and have kids. In this procedure, gametes are put inside a small glass dish where they merge to make a zygote. After the zygote has progressed to the stage of an early embryo, it is put back inside a woman’s body to develop. Today, IVF is a routine (if expensive) procedure and millions of babies have been made with it.
Yet, decades ago, it was considered unethical and impossible and the scientists responsible for the first IVF baby were even accused of “playing God.” Certainly, the process has been streamlined since then.
Originally, IVF was much more demanding and secret. The secrecy was necessary due to how controversial it was back then. Women had to spend two to three weeks at the clinic as inpatients, staying in portable buildings on the grounds of the clinic. They had to collect all their urine during treatment as this was the only way doctors could monitor hormone levels. Patients had to give samples every three hours, even at night.
At one time, the eggs had to be collected through a form of keyhole surgery in which small incisions were made in the body. Now, there is a newer, safer technique that needs only mild sedation. With this new technique, eggs can be collected by sucking them out with a needle under ultrasound guidance. The whole process takes about 30 minutes.[3]
7 Cloning Abnormalities
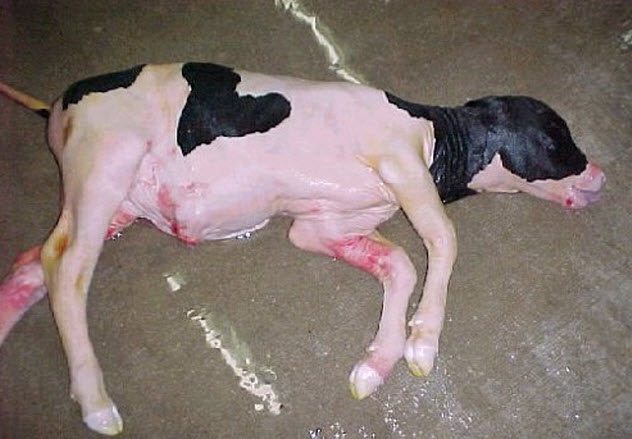
Dolly the sheep, famed as the first clone of an adult mammal, is the only success in a long chain of failures. Of the 277 clones made by the scientists who created her, only Dolly survived to birth.
Failed pregnancies and deaths are a normal part of reproduction. Stillbirths and birth defects can happen no matter how an animal is made. That being said, cloning has a long history of problems.
Some abnormal fetuses develop to term, resulting in abnormalities at birth. The most prominent abnormal phenotype of some clones is “large offspring syndrome.” Calves or lambs with large offspring syndrome are 30–40 percent larger than normal, leading to difficult births. Other health problems include defects in organs, such as the brain, heart, and liver.
Calf and lamb clones that have abnormalities at birth may have health problems for the first few months of their lives. However, after six months, they are indistinguishable in appearance and blood measurements from normally bred animals of the same age.
Cloning’s negative effects on animal welfare caused the European parliament to ban farm animal cloning in 2015. In the US, however, cloning of cattle continues in the hundreds.[4]
6 Resurrection From A Steak

The trouble with cloning cows to make the best beef is that it is impossible to tell if the meat is exceptionally high-quality without killing the cow. Fortunately, there is a way to get around this: Just clone from the steaks.
Only 3 in 10,000 cow carcasses qualify for the best form of rib eye steak, which has plenty of fat in its muscle fibers for flavor but little of the thin layer of back fat that no one wants. When a West Texas A&M University (WTAMU) scientist saw two of these rare rib eyes go by in close succession, he called Dean Hawkins, head of the animal sciences department at the same university.
Only a bit of meat was needed to grow cells, take the DNA, and put it into an egg cell from a cow. One steak came from a castrated bull and the other from a cow that had never given birth. Four clones were made from these two steaks. One (named Alpha) came from the dead bull, while three (named Gamma 1, Gamma 2, and Gamma 3) were cloned from the female cow.
WTAMU bred the clones to pass on the genes for excellent beef. The clones’ 13 calves were the first bovine offspring produced from two cloned carcasses. Seven of those clone offspring were slaughtered. In the evaluation that followed, the carcasses were graded greatly above the industry average.[5]
5 ET And Cows

In the 1970s, American ranchers began bucking the reins of nature’s limits with a technique called embryo transfer (ET). While a cow can normally carry only one embryo at a time, cows that go through ET therapy typically produce six or seven usable embryos. Some can even make as many as 80 to 90 embryos at once.
These embryos are taken out using thin tubes and then put into surrogate cows for gestation. With ET, farmers can produce dozens of calves a year from genetically superior cows without the cows ever having to give birth themselves.[6]
With ET, there is a risk of inbreeding. If farmers choose to breed only offspring from a single cow, that might lower the herd’s genetic diversity and make it more vulnerable to disease. Paradoxically, ET can also help genetic diversity. The USDA has a stockpile of embryos from a variety of livestock breeds at Fort Collins, Colorado.
4 Artificial Twinning

In a way, clones are very natural. When a single fertilized egg splits in two to create two genetically identical individuals, the result is simply identical twins. The simplest way to clone an animal is splitting an early stage embryo, yielding artificial twins. The process of artificial twinning has been done extensively in cows, producing thousands of calves.
The first experiment on artificial twinning was performed by Hans Driesch in 1885. Driesch shook a container holding a two-cell sea urchin embryo, separating the two cells. The two cells then grew into normal, healthy sea urchin larvae. In 1902, Hans Spemann did the same thing in vertebrates using a baby hair noose to split a salamander embryo.
In 2000, a rhesus monkey named Tetra became the first primate cloned via artificial twinning. Tetra was made by splitting an eight-cell embryo into four two-cell pieces.
Though the method was different, Tetra was much like Dolly in that it took a lot of tries to make her. The researchers made 368 embryos by splitting 107 embryos into two or four pieces. Not all the embryos survived. Of all the surrogate mothers, only Tetra’s surrogate had a normal pregnancy.[7]
3 Embryo Screening

Preimplantation genetic diagnosis (PGD) is a type of embryo screening that helps couples going through in vitro fertilization (IVF) to avoid passing on mutations that could cause disability or disease in their children. Generally, PGD involves taking one or a few cells from an embryo made with IVF and testing its gene sequences and chromosomes. Usually, embryos that do not pass these tests are discarded.
Fertility clinics in China are huge and growing. The biggest clinic recorded 41,000 IVF procedures in 2016, which is roughly one-quarter of the yearly number for the whole US. With growth of PGD procedures estimated at 60–70 percent per year, it is expected to catch up in per capita terms in the next few years.
Organized efforts to get rid of genetic disorders and disabilities bring up ethical concerns. Some worry that the push to get rid of disabilities devalues the lives of those who already have them. The cost of PGD also raises concerns about genetic traits further widening the gap between the rich and poor. In China, however, most thoughts are focused on the procedure’s benefits.[8]
Though clinics licensed to do PGD can only use it to prevent serious disease or help infertility treatments, some people ask for more. According to Sijia Lu, chief technology officer of Yikon Genomics, some families ask to weed out the mutation that makes many Asians unable to process alcohol, which could affect the ability to take part in often alcohol-fueled Chinese business lunches. (The company says no.)
2 Artificial Embryos
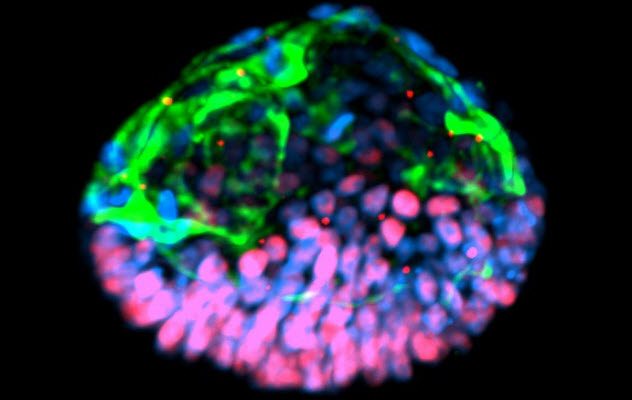
Scientists at the University of Cambridge claimed to have created an artificial mouse embryo using two types of stem cells. One type was embryonic stem cells, while the other was a type that normally makes the placenta.
The scientists put the mix of stem cells onto a 3-D scaffold that mimicked the natural cell support system and helped in the growing cells’ development. Four and a half days later, the cells grew in such a way that they resembled a normal mouse embryo.
Magdalena Zernicka-Goetz, the lead author of the study, told Reuters that the process will allow people to study key events of this stage of human development without actually having to work on embryos. She added that knowing how development normally occurs will allow people to understand why it so often goes wrong.[9]
The proposition raises ethical questions. Currently, scientists can only use discarded human embryos and can only keep them alive for 14 days post-fertilization.
1 The Tale Of The Mouse Princess
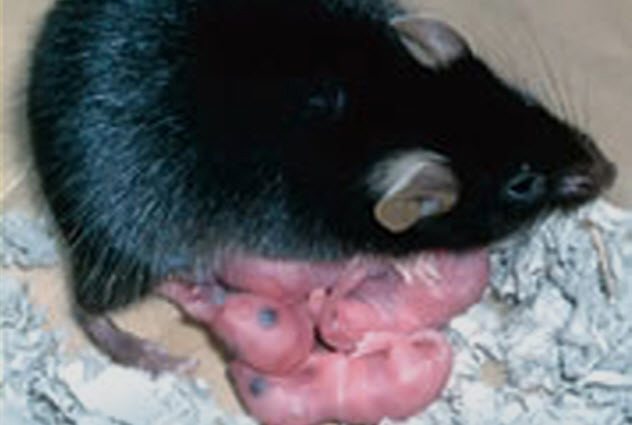
In 2004, scientists at the Tokyo University of Agriculture accomplished a fairy-tale impossibility: a mouse made with no father.
Though mammal egg cells can be artificially made to divide, the resulting fetus always dies in the womb. Scientists have long suspected that this had something to do with imprinting, a process in which some genes are turned on or off during gamete development.
Scientists got around the problem by fusing an immature egg cell, which had not yet gone through imprinting, with a mature one. The immature egg came from a mouse genetically modified to lack certain genes thought to derail development of unfertilized eggs into fetuses.
This happy ending came with its share of struggles. Of 457 egg fusions, only 371 survived to an early clump-of-cells embryonic stage to be put into surrogates. Just 10 live mouse pups were born, and only one survived to adulthood. That lone success was named Kaguya after a Japanese fairy-tale princess found as a baby in a bamboo stump.
According to scientists, it is premature to apply this method to humans because the technique is very labor-intensive and has a high failure rate. It would also involve genetically modifying human eggs, which is generally considered ethically unacceptable.[10]
Jenn Dandy likes biology, parasites, Pokemon, and well-written cartoons. You can find her on her Tumblr blog.
For more bizarre facts about reproduction, check out 10 Amazing Animals That Clone Themselves and Top 10 Fascinating Eggs.