 Mysteries
Mysteries  Mysteries
Mysteries  History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
 Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Space
Space Ten Astonishing New Insights into Alien Worlds
 Weird Stuff
Weird Stuff 10 Bizarre Summer Solstice Rituals Still Practiced Today
 Mysteries
Mysteries Top 10 Haunting Facts About the Ghost Ship MV Alta
 History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
Who's Behind Listverse?

Jamie Frater
Head Editor
Jamie founded Listverse due to an insatiable desire to share fascinating, obscure, and bizarre facts. He has been a guest speaker on numerous national radio and television stations and is a five time published author.
More About Us Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Space
Space Ten Astonishing New Insights into Alien Worlds
 Weird Stuff
Weird Stuff 10 Bizarre Summer Solstice Rituals Still Practiced Today
10 Rare Old Medicines That Had Horrific Side Effects
Medicine has come a long way from the “good old days” of garlic-filled masks and astringent herbal teas. Even in relatively recent times, drugs have been used which have horrific effects on the human body. But—and this is a big but—they can and often do work. At the time, they were the best we had, despite the bizarre ways that they could kill you.
Today’s “Big Pharma” has much more stringent regulations than in the early to mid-1900s. All the items on this list are taken from Grollman and Slaughter’s 12th revision of Cushny’s Pharmacology and Therapeutics, an amazing pharmacopoeia of unusual and bizarre old-world drugs.
10 Metrazol

In 1926, F. Hildebrandt tested a new drug on animals and found two primary clinically significant effects. In high doses, it caused epileptic-like convulsions. In more reasonable doses, it merely stimulated the heart and increased respiration in cases of depressant poisoning (i.e., too much chloroform).
Guess what physicians used it for primarily?
If you said “as an antidote to poisoning,” you would be wrong. (After all, we had pure stimulants for that.) Instead, in 1934, scientist Ladislas J. Meduna pioneered its use in humans to induce convulsions to treat mental illness.
His primary interest was in schizophrenia, for which Metrazol was the first officially recognized treatment. But its use in convulsive therapy expanded to other psychiatric disorders such as depression. In general, patients were sent to a hospital, received Metrazol, and waited for its rapid action to begin. Typically, patients could be discharged within a few hours.
It was considered to be an effective treatment for those diagnosed with psychoses lasting less than three years. At the time, the side effects of this treatment were limited but potentially horrendous. They included spinal fractures, tuberculosis, and brain damage. Luckily, Metrazol quickly fell out of fashion. It was replaced by the “much more efficient” electroconvulsive therapy, which has reduced physical side effects.
Despite its horror, Metrazol is still in use today, just not in hospitals. In labs, it is used to induce convulsions or anxiety in rodents to test treatments for similar disorders. There has also been a recent spike in interest surrounding its potential use in the treatment of Down syndrome, although it wouldn’t be curative.[1]
9 Tribromoethanol

As you might guess by the “ethanol” in the name, tribromoethanol is related to the wonder drink, alcohol. Tribromoethanol has very similar properties, but it is stronger and has a broader range of potential side effects. Cushny’s revised work states that Willstatter first synthesized it in 1923. Later, in 1926, Duisberg used it as an anesthetic.
Rectal administration is remarkably effective. Half the dose is absorbed within 10 minutes and 95 percent within 25 minutes. The effect is predictable: a deep sleep—typically lasting about two and a half hours.
However, there was just one tiny problem: It was almost impossible to alter the hypnotic state. Once you were under, no known drug at the time could wake you. For this reason, tribromoethanol was seldom used. It was just too difficult to control.
Other side effects included injury to the circulatory system, degeneration of the liver and kidneys, slowed metabolism (by around 15 percent), depleted glycogen stores, increased blood sugar levels, and even death.
These days, there are no clinically significant uses for the drug. Instead, it is used to sedate mice in laboratories.[2]
8 Bulbocapnine

This lovely drug was known to be used in the infamous MKUltra program. Similar in structure to apomorphine, bulbocapnine is found in Corydalis cava. It is one of those exciting drugs with a different effect on different animals. In cold-blooded species, it acts in a similar way to morphine by reducing sensitivity to pain and causing sedation.
In warm-blooded animals, however, bulbocapnine induces catalepsy, which is a stiffening of muscles within a given posture that is unable to be moved. The users are frozen in place.
The more highly developed the animal (humans, apes, dogs, etc.), the more pronounced this condition becomes. Also, the higher the dose, the higher the likelihood that narcolepsy will occur. In many cases, bulbocapnine stimulates the bowels, leading to defecation, and invokes the secretion of saliva. Strangely enough, this only occurs in neutered animals.
Luckily, low doses of around 0.1 mg can be tolerated without ill effect, but bulbocapnine has almost no positive clinical uses. Instead, it is used in laboratories and wicked government torture programs. Nowadays, it is being investigated for its potential use in the treatment of Alzheimer’s disease.[3]
7 Picrotoxin

You know a drug is going to be fun when it has “toxin” in the name. Picrotoxin is found in the Anamirta cocculus plant. The symptoms from its use were well-known but delayed.
The first signs of poisoning included vomiting, increased salivation, rapid breathing, and a slowed heart rate with palpitations. Following this was unconsciousness and then violent seizures with periods of respiratory paralysis, which only ceased a few moments later. Well, most of the time. There were instances where patients had died of asphyxia when they failed to restart breathing.
Nevertheless, picrotoxin does have its uses. Traditionally, it was used to treat barbiturate poisoning as it was found to have a stimulating effect on anesthetized patients. It is believed that picrotoxin has a competitive action against the neurotransmitters upon which barbiturates act.
Strangely enough, however, comatose patients can tolerate many times the lethal dose without ill effect. In most patients, picrotoxin is given in 1–3 mg doses at regular intervals. The lethal dose can be as low as 0.357 mg/kg, or 28 mg for an 80-kilogram (176 lb) person.
Even so, some comatose patients have been given doses as high as 300 mg within a day or two with no ill effects. In one reported case, 2.134 grams administered over eight days proved nonfatal.[4]
6 Thymol

Derived from the herb thyme, thymol is one drug that you may recognize as it is one of the constituents in the toothpaste product Euthymol. Traditionally, however, it was used to treat tinea, ringworm, and hookworm infections in humans.
Unfortunately, it has some disturbing side effects when ingested for the treatment of ringworm. Of course, there are the usual poisoning symptoms: nausea, vomiting, and headaches. More unpleasant side effects include a deep depression, paradoxical giddiness, eventual collapse, and possibly death. As always, the key is the dosage: 1–2 grams every couple of hours, followed by a saline purge and emptying of the bowels, is often sufficient and safe.
For the previous skin conditions (tinea and ringworm), a 1:10 preparation of thymol and typically alcohol is applied directly to the skin which eventually rids the patient of the disease. This is primarily because thymol possesses antimicrobial properties. (Thus, it is used in toothpaste.)[5]
However, of all the spices, thyme isn’t the most effective. In order of their power to kill microbes, some favorite herbs with such properties are oregano, clove, coriander, cinnamon, and then thyme. So if you’re thinking of spicing up your lunch when you have a cold, you’d be better off with a carrot and coriander soup than a cinnamon latte.
5 Isonipecaine

In the search for opioid-like painkillers, isonipecaine was developed by and introduced by Eisted and Schaumann in 1939. It is perhaps better known under its other name, pethidine, as a common painkiller used in modern maternity wards for women in labor.
Although isonipecaine is an excellent painkiller known for its mild respiratory depression (compared with morphine) and suppressed vomiting reflex, it is also known for its high rates of euphoria (up to 90 percent) and potential addiction liability when used chronically.
In general, however, its side effects profile is far superior to those of morphine and similar natural opiates. Isonipecaine has little to no effect on respiration, circulation, or metabolic processes.
It is just a shame that it is short-acting (peaking in 45 minutes and lasting around two hours) and less effective than a standard starting dose of morphine. We have also discovered today that isonipecaine is just as addictive despite earlier claims of its lower addiction liability.
It is also grossly toxic in cases of overdoses. If dosed highly multiple times in a short period (3-4 hours), isonipecaine can cause disorientation, rapid heartbeat, and severe respiratory depression.
Its use in labor is well established. However, as it has a less depressive effect on respiration than morphine or diamorphine, isonipecaine is thus comparatively safer for the infant as well as the mother.[6]
Given that it also acts on the smooth muscles within the body, isonipecaine possesses muscle relaxant properties, which can be advantageous in reducing tension and pain during contractions. However, the drug does prolong labor and have an effect on the baby.
4 Intocostrin
Alongside the use of Metrazol in electroconvulsive therapy was another critical drug: introcostrin.
It’s worth noting that introcostrin is derived from curare, which was used by native South Americans in a poison concoction to lace the tips of their arrows when hunting. Curare stops all voluntary movement.
As it was put by Cushny et al.: “The muscles give way one after another until the animal lies helpless on the ground . . . and becomes totally paralyzed.”
Eventually, it suffocates the victim when the respiratory system ceases to function. In essence, this is a fatal form of locked-in syndrome. So yeah, deadly stuff. Fun fact: Oral ingestion is practically harmless. So you can suck the poison out of a wound and have a chance of survival.
Curare has minimal therapeutic use. It is entirely dependent on getting a dose high enough to relax muscles but not freeze breathing. This is a tricky process, so it is often avoided in favor of intocostrin.
As it was much easier to control and dose, intocostrin was chiefly used to relax patients undergoing convulsive or electroconvulsive therapy. The drug reduced the severity of the violent convulsions. This was generally thought to lower the prevalence of spinal fractures, a significant issue for such patients. Intocostrin also reduces spasms and is used as an adjunct agent for anesthesia.[7]
3 Dinitrophenol

This is one of those drugs that seemed like a great idea at the time. However, it soon became apparent that there were significant issues with its use. Dinitrophenol was first discovered during World War I in munitions workers who died from contact with the chemical. You’d think that would be the end of the matter, but the drug was quickly investigated to see if there were any therapeutic uses.
Applied at a dose of 3–5 mg per kilogram of body weight, dinitrophenol was found to increase metabolism by 20–30 percent for days on end as a result of increased oxygen consumption. This seemed to be a tremendous potential treatment for obesity.
However, as the dose slowly crept up, it could begin to induce profuse sweating and an increase in body temperature by up to 3 degrees Celsius (5.4 °F). In toxic doses, this was followed by numerous symptoms including rapid breathing.
This latter problem and the increased oxygen requirement eventually meant that the patient’s breathing couldn’t possibly keep up with and supply the body’s oxygen needs. Hypoxia could develop along with a fever of 43 degrees Celsius (109 °F) or more—and that’s just the start. Common side effects at standard doses included a range of internal and external illnesses which could easily result in death.[8]
Although used for a time to treat obesity, dinitrophenol quickly fell out of favor due to its highly toxic chronic effects and potential for fatal syndromes. In fact, its primary use these days is as a pesticide or as part of an explosive mixture called shellite.
2 Ergot

Ergot is an infamous fungus that grows on rye and other grasses such as wheat. This fungus is known to cause the notorious gangrenous condition of ergotism (aka “St. Anthony’s Fire”). This may be partially responsible for the witch hunts in the Middle Ages as symptoms can include psychoses and delirium.
Despite this, it did and still does have its therapeutic uses.[9] Ergot is powerful at inducing contractions of the uterus, inducing labor, or causing abortions.
However, it is preferable that it only be used after the second stage of labor, after the placenta has been delivered, to ensure that the child does not suffocate. When applied at this stage, ergot reduces bleeding and prevents postpartum hemorrhaging.
Indeed, it was believed to be useful in all cases of internal hemorrhaging since it contracts the walls of blood vessels and reduces bleeding. However, prolonged treatment can quickly lead to the development of gangrene. Even so, ergot or a derivative may be useful in the treatment of parkinsonism.
1 Santonin
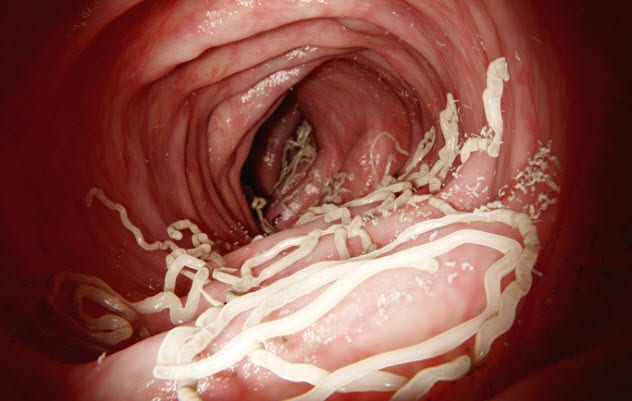
Bitter to the taste, santonin, a drug developed in the early 1800s, used to be the primary treatment for roundworms and pinworms before being replaced by safer compounds. It continued for a while longer as a treatment for whipworm. However, the drug was completely ineffective against tapeworm.
The side effects were nasty but bizarre and somewhat humorous.[10] For example, patients reported that their vision was altered. Not in any particularly bad way, but everything took on a blue tint.
This was very brief before another visual disturbance took effect. Bright objects appeared to have illustrious yellow auras, blues turned to greens, and the previous blues would get darker and darker until they were indistinguishable from black. The more santonin that was ingested, the more vivid and intense these perceptions were.
In addition, patients experienced nausea, vomiting, and some confusion. In higher doses, convulsions occurred with the potential for asphyxia. The drug is also excreted in almost every way possible: in the feces with the worms, in the urine which is turned neon yellow, and even in sweat which takes on a yellow tinge.
The theory of how santonin kills the parasites can be summarized simply: It kills them before it kills you.
Freelance researcher and writer on a range of topics. I’m always looking for new work, so feel free to contact me at [email protected] with any ideas!
For more bizarre facts about old medicines, check out Top 10 Corpse Medicines That Turned Patients Into Cannibals and 10 Wacky Vintage Cures Con Artists Sold To The Masses.



![9 Sinister Facts About The Dark Side Of Instagram [WARNING: Disturbing] 9 Sinister Facts About The Dark Side Of Instagram [WARNING: Disturbing]](https://listverse.com/wp-content/uploads/2019/10/proxy.duckduckgo-150x150.jpg)




