 Weird Stuff
Weird Stuff  Weird Stuff
Weird Stuff  Animals
Animals 10 Inspiring Tales of Horses Being Human
 Mysteries
Mysteries Top 10 Haunting Facts About the Ghost Ship MV Alta
 History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
 Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
 Weird Stuff
Weird Stuff 10 Times Real Laws Were Based on Bizarre Hypotheticals
 Animals
Animals 10 Inspiring Tales of Horses Being Human
 Mysteries
Mysteries Top 10 Haunting Facts About the Ghost Ship MV Alta
Who's Behind Listverse?

Jamie Frater
Head Editor
Jamie founded Listverse due to an insatiable desire to share fascinating, obscure, and bizarre facts. He has been a guest speaker on numerous national radio and television stations and is a five time published author.
More About Us History
History 10 Surprising Stories About the Texas Rangers
 Humans
Humans 10 Philosophers Who Were Driven Mad by Their Own Theories
 Miscellaneous
Miscellaneous 10 Video-Game-Worthy Weapons and Armors from History
 Weird Stuff
Weird Stuff 10 Psychics Who Accurately Predicted Wartime Events
 The Arts
The Arts 10 Pieces of Art Inspired by a Broken Heart
 Health
Health 10 Science Fiction-Sounding New Medical Treatments
 History
History 10 Surprising Facts About the Father of Submarine Warfare
10 Chemical Reactions That Changed The World
Chemistry surrounds us every day. From cooking our food to driving our cars to our very own bodily metabolism, we cannot escape the constant rearrangement of atoms and exchange of energy that is chemistry.
Though this constant shifting of atoms forms a nearly unnoticeable background in our daily lives, there are some reactions that have truly changed, or will change, how mankind has lived. Some because of what we could do with them. Others because of what they showed us. But all became landmarks in mankind’s journey.
Here are 10 chemical reactions that changed the world.
10 Ammonia Synthesis

Nitrogen is one of the most important elements for life, perhaps behind only carbon. It is a key component in DNA, RNA, proteins, and chitin (a biological polymer similar to cellulose found in fungi, insects, lobsters, shrimp, and some fish). Nitrogen is also one of the most plentiful elements on Earth, making up approximately 78 percent of the Earth’s atmosphere. However, nitrogen in the atmosphere exists in the form of N2, which is highly unreactive and not useful for most life-forms.
Therefore, nitrogen must be fixed by converting it to more reactive forms, such as ammonia, nitrates, and nitrites. In nature, this is usually done by specialized bacteria. These bacteria form a symbiotic (meaning that both organisms benefit) relationship with many plants, living in nodules in the roots.
However, not all plants form this relationship. Especially in the case of commercial farming, crops such as corn do not fix nitrogen but absorb it from the soil. If a crop that does not fix nitrogen is grown for several seasons, it will be necessary to add fertilizer. However, few naturally occurring materials have enough nitrogen to act as a fertilizer. Therefore, to meet increasing demands for food, it was necessary to find a better way to produce nitrogen fertilizer.
The Haber-Bosch process was the first step. Developed by Fritz Haber and Carl Bosch in 1918, the process used both high temperatures and high pressures and an iron catalyst to produce a large amount of ammonia from gaseous hydrogen and nitrogen.[1]
As the ammonia was relatively cheap to produce, it became a viable alternative to natural fertilizers. Today, ammonia is the second most produced chemical by tonnage, after only sulfuric acid.
9 Polymerization Of Polyethylene

Plastic revolutionized the world. As they are easily molded, resistant to both heat and chemical attack, and cheap to make, plastics have become a ubiquitous material in everyday life—especially polyethylene. Coming in a variety of forms such as high-density polyethylene and low-density polyethylene, it is used in plastic bags, milk bottles, and even bulletproof vests.
Polyethylene was accidentally discovered in 1933 by two scientists working for the Imperial Chemical Industries Research Laboratory while trying to react ethylene and benzaldehyde. Instead, a waxy material was discovered, which was found to be a polymer of ethylene. A polymer is a substance that is made up of many repeating units. Other polymers include cellulose and DNA.
By 1937, the material had been developed as a film and was used as insulation for wires and radar components by the British in World War II.[2] As it made electrical components light enough to place in planes, its structure and manufacture were a highly guarded secret. Today, polyethylene is the most produced plastic in the world, with 81.8 million tons made in 2015 and almost 100 million tons estimated to be made in 2018.
8 Combustion Of Hydrogen

In the late 1700s, chemistry was an underdeveloped science. Most chemistry was rooted in the Greek elements of air, water, earth, and fire, with additions made as necessary to explain observations.
One of the most notable additions was phlogiston. Developed by Georg Stahl, the concept stated that all inflammable substances contained a fire element called phlogiston. Upon combustion, this phlogiston would be lost to the air. This seemed to explain why burned charcoal weighed less than the original charcoal. However, this theory failed to explain why some substances, such as phosphorus and sulfur, gained mass during combustion.
Enter Antoine Lavoisier, a French scientist who was very skeptical of the theory of phlogiston. In perhaps his most famous experiment, he combusted what was known as inflammable air (hydrogen gas) with normal air. The product was water. Lavoisier believed that water must be a combination of a substance in the air (which he called oxygen) and the inflammable air.
He further supported his hypothesis by decomposing water into oxygen and hydrogen. In 1789, Lavoisier’s new system of chemistry was fully published in his textbook Traite elementaire de Chimie (“Elements of Chemistry”), which abandoned the Greek system and laid the foundation for modern chemistry.[3]
7 Reduction And Oxidation Of Zinc And Silver
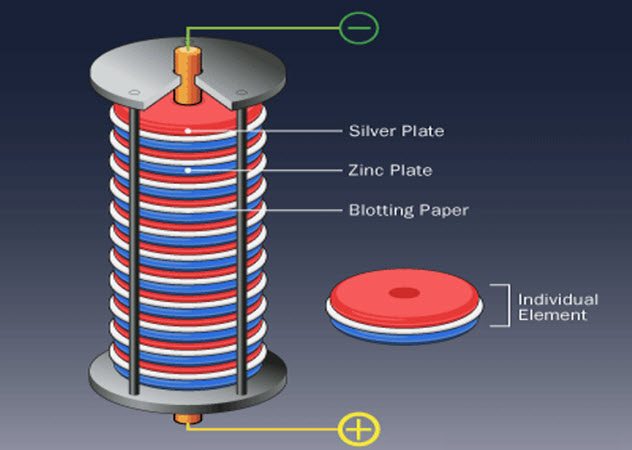
When Alessandro Volta was born in Como, Italy, in 1745, electricity was a poorly understood phenomenon. It was known that electricity could be conducted and that it came in two forms (what would later come to be known as positive and negative).
Shortly after Volta’s birth, it was demonstrated by Benjamin Franklin that lightning was actually electricity. Although Volta did not have a university-level education, he became widely known as a scientist in his day. In 1775, he developed the perpetual electrophorus, an improvement on previous versions of the electrophorus. However, another invention was to be his most important.
In 1780, scientist Luigi Galvani made the claim that animal muscles produced electricity when they contracted. He called this “animal electricity” and believed it to be different than regular electricity.
Volta disagreed, noting that Galvani’s frog legs had been connected to two different metals during the experiments. Volta proceeded to demonstrate that by stacking alternating metal discs of silver and zinc, with brine-soaked cloths between each disc, he could create a steady electric current with no animals.
However, it was instantly recognized that Volta’s invention was far more useful than just settling his dispute with Galvani. All previous sources of electricity could only generate it in bursts. By generating a steady current, Volta’s invention allowed for more rigorous study, laying the foundation for the revolutionary work of Faraday in electromagnetism.[4]
6 Synthesis Of Urea

Vitalism was a theory that living systems were governed by completely different principles than nonliving systems. Additionally, it was believed that the components that made up living systems could not be made of nonliving components. This belief was widely held in the 19th century and was used to explain why many living systems seemed incomprehensible compared to nonliving systems.
However, German scientist Friedrich Wohler changed that. Already known for his isolation of pure aluminum in 1825, Wohler was working on trying to synthesize ammonium cyanate in 1828. However, when he reacted silver cyanate and ammonium chloride in an attempt to produce the ammonium cyanate, he instead produced white crystals. He later identified the substance as urea.
Urea had been isolated in 1773 by French chemist Hilaire-Marin Rouelle. This meant that Wohler had just synthesized an organic compound, which disproved one of the basic tenets of vitalism. Wohler’s work would go on to lay the foundation for the field of organic chemistry.[5]
5 PCR
 Polymerase chain reaction (PCR) is by far the most complicated reaction on this list but potentially the most useful and exciting. PCR was invented in 1983 by Kary Mullis, who eventually won a Nobel Prize for his work.
Polymerase chain reaction (PCR) is by far the most complicated reaction on this list but potentially the most useful and exciting. PCR was invented in 1983 by Kary Mullis, who eventually won a Nobel Prize for his work.
The process works by heating DNA so that it separates into two single strands. (DNA is double-stranded.) Then primers can be attached to the individual DNA strands. Enzymes called DNA polymerases attach at the primer sites and replicate the rest of the strand of DNA. This process can be repeated many times, with each iteration theoretically doubling the number of exact DNA copies.[6]
The ability to replicate DNA opened doors in many fields. It allowed forensic scientists to apply genetic techniques even if there was only a small amount of genetic material left at the crime scene. In medicine, it is useful in helping to identify the cause of infections. In research, it was an essential technique used during the sequencing of the human genome.
Beyond these, it is now a ubiquitous technique in biology and biochemistry labs worldwide.
4 Fat Hydration

Do you have a jar of Crisco in your pantry? Would it surprise you if I told you that Crisco was the result of one of the world’s most revolutionary advances in food technology?
It all begins with the difference between animal fats and plant fats. Fats in animals tend to be saturated, meaning that all the carbon in the fat is bonded to the maximum number of atoms. Fats in plants tend to be unsaturated, meaning that some of the carbon in those fats was not bonded to the maximum number of atoms.
In 1902, Wilhelm Normann developed a process that made it possible to add hydrogen to unsaturated fats, which would turn them into saturated fats or at least more highly saturated fats. In 1909, Procter & Gamble acquired Normann’s patent. Two years later, they released Crisco, a shortening made mostly of hydrogenated cottonseed oil, which was cheaper than standard lard.
However, that was only the beginning. By 1979, approximately 60 percent of all fats consumed in the United States had been hydrogenated. But there was a dark side to hydrogenation. Natural unsaturated fatty acids occur almost exclusively in the cis configuration, which causes the fat molecules to have a bend or kink in them and not be able to fit together as well. This is the reason that most unsaturated fats are liquids.[7]
However, during hydrogenation, some unsaturated fatty acids take up the trans configuration. Starting in the 1990s, research showed that high consumption of trans fats resulted in adverse health effects. Shortly thereafter, the FDA began to regulate the amount of trans fats in food and some localities even banned these substances. This led to the eventual decline of hydrogenated fats.
3 Ozone Destruction

Mechanical refrigeration technology had been in common usage since at least the 1870s. However, there was a huge problem that limited the technology at the time. Most of the refrigerants (substances used to move heat from inside the refrigerators to outside) were either highly toxic or highly flammable. Unfortunately, it was relatively common for people to die due to leaking refrigerant.
To solve this problem, Frigidaire, Dupont, and General Motors joined forces to find a refrigerant that would be much safer. The result was Freon, a mixture of a class of chemicals called chlorofluorocarbons (CFCs). Freon was so safe that its inventor both directly inhaled it and then breathed it out onto a candle in front of the American Chemical Society.[8]
However, CFCs had an unknown problem at the time. With so many refrigerators using CFCs, the chemical quickly reached significant levels in the atmosphere. When exposed to ultraviolet light in the upper atmosphere, CFCs would often give off a chlorine atom.
Chlorine is highly reactive and catalyzes the breakdown of ozone (O3) to molecular oxygen (O2). As catalysts only speed up the rate of a reaction and are not consumed in the reaction, one molecule of a CFC could lead to the destruction of thousands or even millions of molecules of ozone, causing large-scale depletion of the ozone layer.
Today, CFCs are highly regulated by the Montreal Protocol and are no longer used as a refrigerant. They have been replaced by a similar class of compounds known as hydrofluorocarbons (HFCs). While HFCs also have drawbacks (they are a very strong greenhouse gas), there have been no newly developed refrigerants that are both nontoxic and nonflammable.
2 Water With Carbon Dioxide
 Carbon dioxide is perhaps best known for its role as a greenhouse gas. As levels of carbon dioxide in the atmosphere rose, so did average global temperatures. However, there is a second dark side to carbon dioxide and it happens every day when we drink a soda.
Carbon dioxide is perhaps best known for its role as a greenhouse gas. As levels of carbon dioxide in the atmosphere rose, so did average global temperatures. However, there is a second dark side to carbon dioxide and it happens every day when we drink a soda.
Carbon dioxide reversibly reacts with water to form carbonic acid. Some of this carbonic acid then breaks down into bicarbonate and then carbonate ions while releasing H+ (releasing H+ is the defining characteristic of acids called Bronsted-Lowry acids). This acid is part of the sharp sensation of a fresh soda.
However, carbon dioxide in the atmosphere can react the same way with the water in the ocean. In fact, the ocean absorbs approximately one-quarter of the carbon dioxide released every year.
As a result, the pH of surface ocean waters has declined by approximately 0.1 pH units since the beginning of the industrial revolution, which is a nearly 30 percent increase in acidity. While this increase in acidity benefits some organisms like algae and seagrasses, it is harmful to many organisms such as oysters, clams, shellfish, and coral.[9]
One UN report estimated that ocean acidification could cost as much as $1 trillion by 2100.
1 Saponification

It is fairly common knowledge that oil and water do not mix. The reason for this has to do with a concept called polarity. Simply put, water molecules are polar and oil molecules are not. As water molecules are polar, it is more favorable for them to be next to each other than next to a nonpolar oil molecule. However, as any cook knows, this can provide a problem when it comes to cleaning the dishes. The grease will not mix with the water and stays on the dish.
The answer is soap. Soap molecules have both polar and nonpolar parts to them. The polar part mixes with the water while the nonpolar part mixes with the oil, which allows the oil to form small droplets in the water that are more easily removed.
The reaction used to create soap is the saponification reaction. Originally, soap was made by heating salt, ashes, and animal fats together in water. The first known soaps were made using this process in Babylon in 2800 BC. Today, soap is made by reacting either sodium hydroxide or potassium hydroxide with fatty acids (which are derived from fat molecules).[10]
However, for purposes other than personal hygiene, soaps have largely been superseded by detergents. These cleansing agents are similar to soaps but are usually derived from petrochemicals and have several advantages over soaps. They tend to last longer without decomposing. They also tend to be more soluble in cold water or hard water (water that has a relatively high calcium content), which means we aren’t as likely to see that nasty soap scum.
Just another student. I also like fire. More than I probably should.
Read more fascinating facts about chemicals on 10 Ridiculously Dangerous Chemicals and Top 10 Amazing Chemical Reactions.







